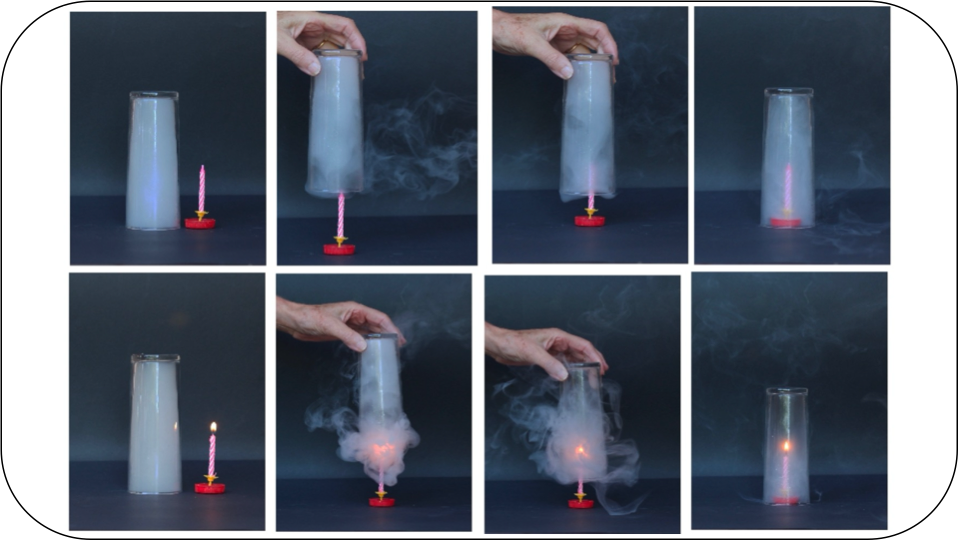
Water rising through the interior of a vessel that covers a candle over a plate with water: an experiment poorly understood, analysis of a persistent error and proposals to overcome it
Downloads
- PDF (Español (España)) 801
- EPUB (Español (España)) 99
- VISOR (Español (España))
- MÓVIL (Español (España))
- XML (Español (España)) 90
DOI
https://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2024.v21.i2.2203Info
Abstract
The experiment of covering a lit candle in a water basin is an educational resource widely used to show students what happens when the pressure inside a container becomes lower than atmospheric pressure. Although the experiment is simple, quick, and easily reproducible, it is likely not to be understood by most of the audience, as inferred from the analysis of the responses given by students and university graduates when asked to explain why water enters the glass as soon as the candle flame weakens. In the second part of this article, and to facilitate the understanding of this experiment and analyze certain aspects of the combustion of a candle, a collection of complementary experiments is proposed that helps comprehend what takes place within the glass.
Keywords
Downloads
How to Cite
License
Copyright (c) 2024 Antonio Tomás Serrano, Rafael Garcia-Molina

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Require authors to agree to Copyright Notice as part of the submission process. This allow the / o authors / is non-commercial use of the work, including the right to place it in an open access archive. In addition, Creative Commons is available on flexible copyright licenses (Creative Commons).

Reconocimiento-NoComercial
CC BY-NC
References
ACS (2023). Flame out carbon dioxide experiment. American Chemical Society. https://www.acs.org/education/whatischemistry/adventures-in-chemistry/ experiments/flame-out.html
Beyler, C. (2016). Flammability limits of premixed and diffusion flames, Cap. 17, pp. 529-553 en SFPE Handbook of Fire Protection Engineering. 5ª ed., M. J. Hurley, D. Gottuk, J. R. Jr. Hall, K. Harada, E. Kuligowski, M. Puchovsky, J. Torero, J. M. Jr. Watts, C. Wieczorek (Eds.). Gaithersburg: SFPE. http://doi.org/10.1007/978-1-4939-2565-0
Birk, J. P. y Lawson, A. E. (1999). The Persistence of the Candle-and-Cylinder Misconception. Journal of Chemical Education, 76(7), 914-916. https://doi.org/ 10.1021/ed076p914
Bueno Garesse, E. (2004). Aprendiendo química en casa. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 1(3), 45-51.
Campanario, J. M. y Otero, J. C. (2000). Más allá de las ideas previas como dificultades de aprendizaje: las pautas de pensamiento, las concepciones epistemológicas y las estrategias metacognitivas de los alumnos de ciencias. Enseñanza de las Ciencias, 18(2), 155-169. https://ensciencias.uab.cat/article/view/v18-n2-campanario-otero/ 1943
Cooper, E. K. (1963). Discovering Chemistry. London: Butterworth.
Crujeiras Pérez, B. y Jiménez Aleixandre, M. P. (2015). Desafíos planteados por las actividades abiertas de indagación en el laboratorio: articulación de conocimientos teóricos y prácticos en las prácticas científicas. Enseñanza de las Ciencias, 33(1), 63-84. https://ensciencias.uab.cat/article/view/v33-n1-crujeiras-jimenez/1469-pdf-es
Fang, C. H. (1998). A Simplified Determination of Percent Oxygen in Air. Journal of Chemical Education, 75, 58-59. https://doi.org/10.1021/ed075p58
García-Rodeja Gayoso, I. y Sesto Varela, V. (2016). ¿Por qué sube el agua? Un estudio comparativo del desempeño en el uso de pruebas. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 13(2), 215-229. http://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2016.v13.i2.01
Girault, I., D’Ham, C., Ney, M., Sánchez, E. y Wajeman, C. (2012). Characterizing the experimental procedure in science laboratories: a preliminary step towards students experimental design. International Journal of Science Education, 34(6), 825-854. http://doi.org/10.1080/09500693.2011.569901
IUB (2017). Sodium Hydroxide and Carbon Dioxide. Indiana University Bloomington. https://www.chem.indiana.edu/wp-content/uploads/2017/11/17-3-Sodium-Hydroxide-and-Carbon-Dioxide.doc
Krnel, D. y Glazar, S. A. (2001). Experiment with a Candle without a Candle. Journal of Chemical Education, 7, 914. https://doi.org/10.1021/ed078p914
Matute, S., Iglesias, P., Gutiérrez, O., Capote, T., Rojas, J. y Durán, R. (2013). Representaciones mentales en el aprendizaje del concepto combustión. Educere, 17(57), 309-318. https://www.redalyc.org/articulo.oa?id=35630152015
Peckham, G. D. (1993). A new use for the candle and tumbler myth. Journal of Chemical Education, 70(12), 1008-1009.
Roth K. (2003). Alle Jahre wieder - die Chemie der Weihnachtskerze. Chemie in unserer Zeit, 37, 424-429. http://doi.org/10.1002/ciuz.200390086
Que, R., Sha, S., Shen, L. y Xiong, Y. (2020). Changes of CO2, concentration and heat illustrate why the flame is extinguished in the candle-and-cylinder experiment. Journal of Chemical Education, 97(4), 1195-1197. https://doi.org/10.1021/acs.jchemed.9b00833
Riveros, H. G. (2012). Popular Explanations of Physical Phenomena: Broken Ruler, Oxygen in the Air and Water Attracted by Electric Charges. European Journal of Physical Education, 3, 52-57. http://eujournal.org/index.php/EJPE/article/view/111
Rudel, D. I. (2010). Science Myths Unmasked: Exposing misconceptions and counterfeits forged by bad science books (Vol. 1: Earth and Life Science). Gadflower Press. ISBN: 9781935776024.
Sesto Varela, V. y García-Rodeja Gayoso, I. (2017). Estudio sobre la evolución de los modelos mentales de estudiantes de 4º de ESO cuando observan, reflexionan y discuten sobre la combustión. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 14(3), 521-534. https://doi.org/10.25267/Rev_Eureka_ensen_divulg_ cienc.2017.v14.i3.02
Shakhashiri, B. Z. (1985). Ch. 6.13 Combustion of a candle in air, en Chemical Demonstrations: A Handbook for Teachers of Chemistry, Vol. 2. Madison: University of Wisconsin Press.
Tomás Serrano, A. y García Molina, R. (2015). Experimentos de Física y Química en tiempos de crisis. Murcia: Editum. ISBN: 978-84-84-16038-96-1.
Tomás-Serrano, A., y García-Molina, R. (2023). Una contribución para dilucidar las principales causas del ascenso de gua en un vaso invertido sobre la llama de una vela. Anales de Química, 119 (2), 180-188.
Vázquez Dorrío, J. B., García Parada, E. y González Fernández, P. (1994). Introducción de demostraciones prácticas para la enseñanza de la Física en las aulas universitarias. Enseñanza de las Ciencias, 12 (l), 63-65. https://ensciencias.uab.cat/article/view/ v12-n1-vazquez-garcia-gonzalez/2393
Vera, F., Rivera, R. y Núñez, C. (2011). Burning a Candle in a Vessel, a Simple Experiment with a Long History. Science & Education, 20, 881-893.
Watson J. R., Prieto T. y Dillon J. S. (1997) Consistency of students' explanations about combustion. Science Education, 81(4), 425-443.
Wisniak, J. (2001). Candle – A light into the past. Indian Journal of Chemical Technology, 7, 319-326.