A simple low-cost UV light photometer for experimental teaching of the Beer-Lambert law
Downloads
- PDF (Español (España)) 667
- EPUB (Español (España)) 65
- VISOR (Español (España)) 32
- MÓVIL (Español (España)) 31
- XML (Español (España)) 72
DOI
https://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2024.v21.i3.3401Info
Abstract
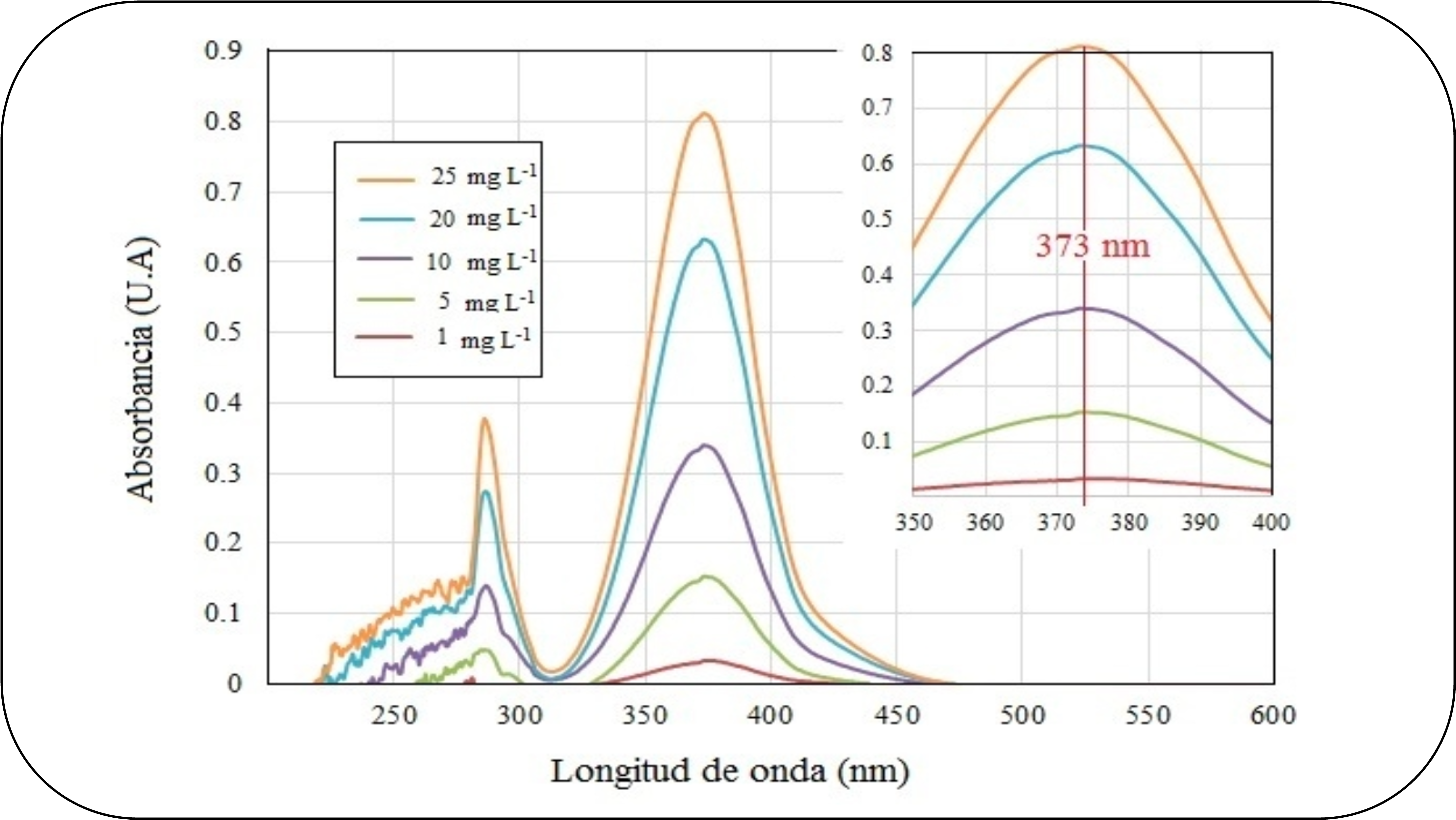
This article presents a low-cost photometer for experimental teaching designed to determine hexavalent chromium in aqueous solutions based on the detection of yellow chromate ions with ultraviolet light at 373 nm. This procedure replaces the colorimetric method that uses a spectrophotometer for the detection of the red-violet 1.5-diphenylcarbazide complex at 540 nm. To demonstrate the operation of the photometer, the Beer-Lambert law was evaluated using the linear regression technique, and the absorbance values obtained by the photometer were compared with those of a Shimadzu UV-2600 series UV-Vis spectrophotometer in five samples of hexavalent chromium in the range of 1 – 25 mg L-1. The experimental results demonstrated the linearity required by the Beer-Lambert law with a determination coefficient of 0.9988, and similarity between both analytical instruments with a relationship of 1.0041 between the absorbance values with a determination coefficient of 0.9993. In conclusion, the low-cost photometer is an excellent proposal for teaching the Beer-Lambert law in the classroom because it reduces the formation of waste, and dispenses with the reagents of the colorimetric method.
Keywords
Downloads
Supporting Agencies
How to Cite
License
Copyright (c) 2024 Evelyn Toque-Huaman, Julio David Gonzales Balladares

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Require authors to agree to Copyright Notice as part of the submission process. This allow the / o authors / is non-commercial use of the work, including the right to place it in an open access archive. In addition, Creative Commons is available on flexible copyright licenses (Creative Commons).

Reconocimiento-NoComercial
CC BY-NC
References
Almonte, K. B., Amador, F. C., Hernández, A., Trujillo, M. (2018). Construcción de un fotómetro con leds: Estudio comparativo. Tendencias en Docencia e Investigación en Química, 4(4), 61-70. http://hdl.handle.net/11191/8193
Cid, R., González-Fernández, D. (2020). Una aproximación a la espectrometría en educación secundaria. Anales de la Química, 116(1), 25-29. https://analesdequimica.es/index.php/AnalesQuimica/article/view/1309
Crocombe, R. A. (2018). Portable Spectroscopy. Applied Spectroscopy, 72(12), 1701-1751. https://doi.org/10.1177/0003702818809719
Gonzales-Balladares, J. D. y Toque-Huaman, E. (2024). Diseño de un fotómetro con luz UV de bajo costo (V1). Mendeley Data. https://doi.org/10.17632/4jszdxsnws.1
Heredia Avalos, S. (2006). Experimentos de química recreativa con sulfato de cobre pentahidratado. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 3(3), 467-484. https://revistas.uca.es/index.php/eureka/article/view/3851/3429
Montoya, E., Baltuano, Ó., Arbildo, A. (2013). Espectrómetro para radiación visible hecho en casa, de bajo costo y altas prestaciones. Revista de la Sociedad Química del Perú, 79(1), 80-91. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1810-634X2013000100011
Moreira, A. F., Santos, S. D., Junior, A. C. (2016). Construção e caracterização de um fotômetro destinado ao uso de aulas experimentais de química sobre a lei de Beer-Lambert. Holos, 2, 142-151. https://doi.org/10.15628/holos.2016.4016
Onchoke, K. K., y Sasu, S. A. (2016). Determination of hexavalent chromium (Cr(VI)) concentrations via ion chromatography and UV-Vis spectrophotometry in samples collected from nacogdoches wastewater treatment plant, east Texas (USA). Advances in Environmental Chemistry, 2016, 1-10. https://doi.org/10.1155/2016/3468635
Poh, J-J., Wua, W-L, Gohb, N., Tana, S., y Gana, S. (2021). Spectrophotometer on the go: The development of a 2 in 1 UV–Vis portable Arduino based spectrophotometer. Sensors and Actuators A: Physical, 325, 1-8. https://doi.org/10.1016/j.sna.2021.112698
Ramírez-Silva, M. T., y Rojas-Hernández, A. (2012). La enseñanza experimental de la química general y la química analítica desde la ENEP-Cuautitlán de la UNAM y la UAM-Iztapalapa en el último cuarto del siglo XX. Educación Química, 23(1), 136–140. http://dx.doi.org/10.1016/S0187-893X(17)30145-3
Ramos, L., Charca, J. R., Veleto, M. I. (2023). Prototipo de un espectrofotómetro modular para la enseñanza de la química. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 20(2), 2401. https://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2023.v20.i2.2402
Sanchez-Hachair, A., Hofmann, A. (2018). Hexavalent chromium quantification in solution: Comparing direct UV-visible spectrometry with 1,5-diphenylcarbazide colorimetry. Comptes Rendus Chimie, 21(9), 890-896. https://doi.org/10.1016/j.crci.2018.05.002
Skoog, D. A., Holler, F. J., Crouch S., R. (2009). Princípios de Análise Instrumental. 6th ed., Porto Alegre: Bookman / Grupo A.
Swinehart, D. F. (1962). The Beer-Lambert Law. Journal of Chemical Education, 39(7), 333. https://doi.org/10.1021/ed039p333